"Photosynthesis - how was it again?"
There are many reasons to be interested in photosynthesis: Perhaps you are studying the subject at school right now? Or you want to refresh what you once learned in biology classes. You may just want to understand how light leads to growth in plants.
Then this article is just right for you.
Perhaps you have already asked yourself:
Why is a plant the way it is?

To approach the answer, let's look at photosynthesis from a slightly different perspective:
1) Photosynthesis is the splitting of water by light
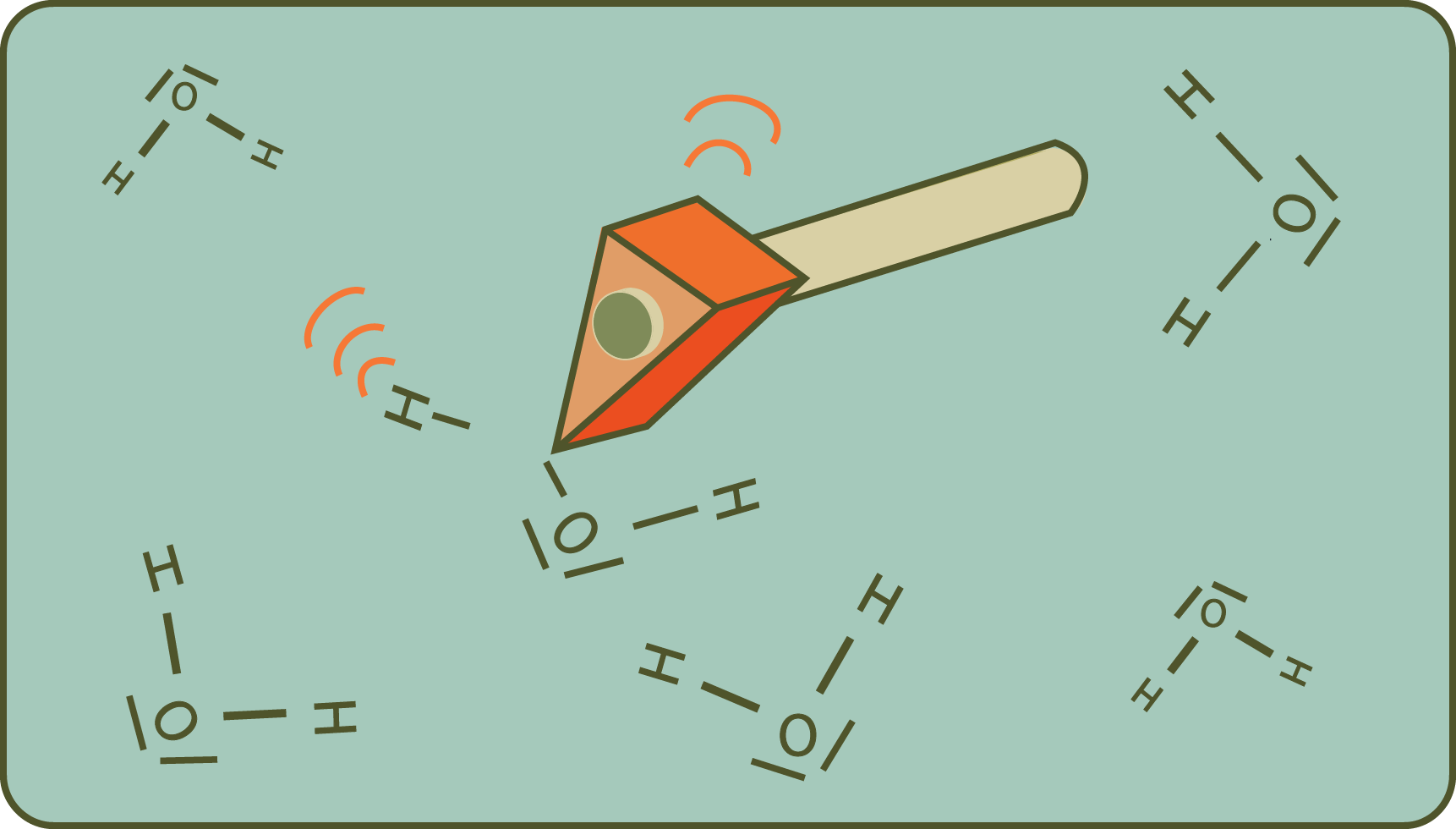
What? Sounds strange, doesn't it? But one by one.
In school you usually learn first that photosynthesis turns light energy, water and carbon dioxide into sugar and oxygen.
The bottom line is also correct, but let's start a bit different:
If you live on a planet that is hit by huge amounts of light energy like the Earth, it is certainly a good idea to use this energy to build molecules. Before this was possible through the evolution of living things, organisms (e.g. bacteria) had to use inorganic substances such as iron sulfide or other as an energy form.
But how can you use solar energy to build cells out of it? Well, for example by somehow managing to create longer-chain carbon skeletons from the carbon dioxide in the atmosphere. That's pretty much everything in a cell.
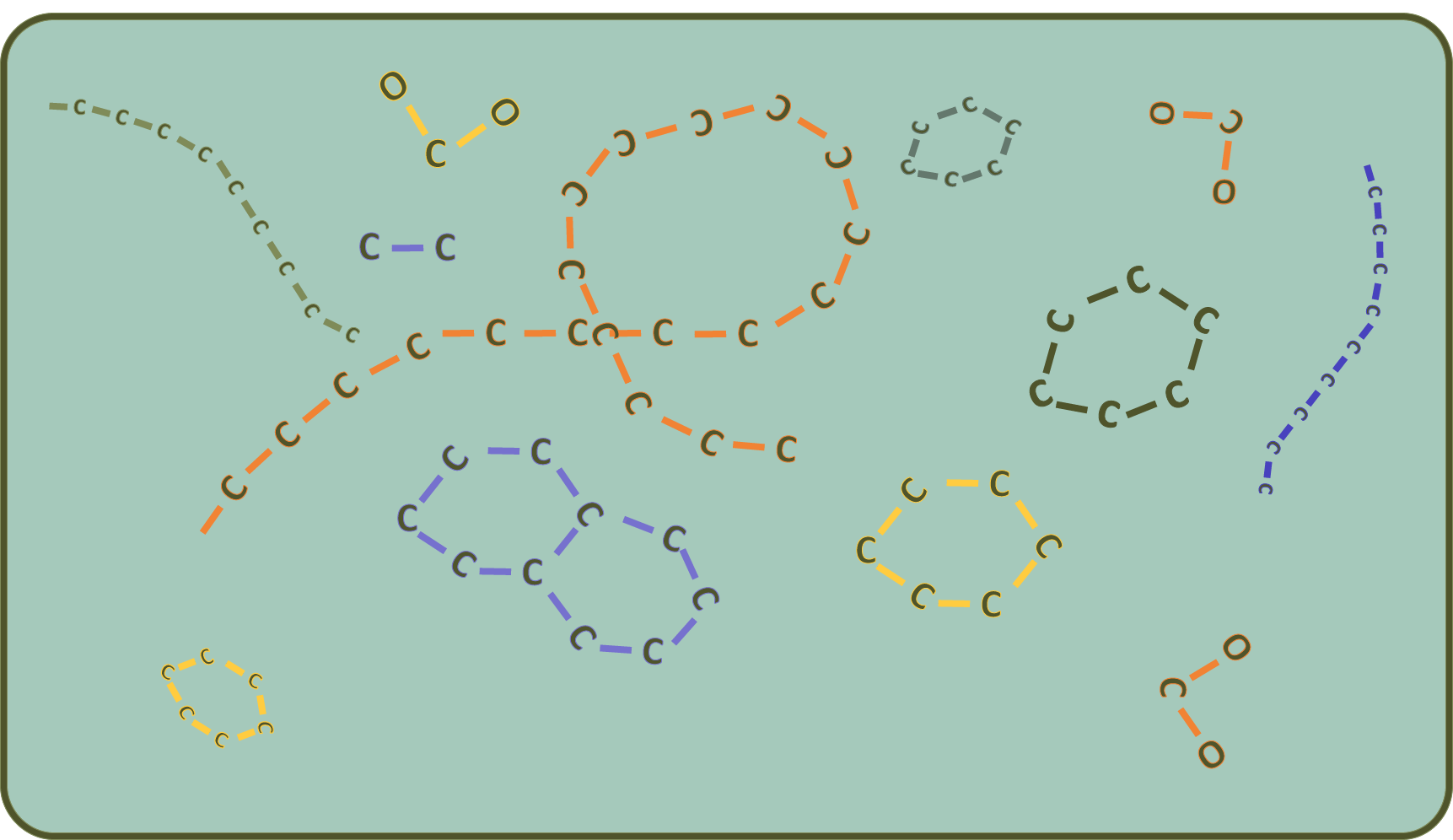
So that's exactly what photosynthesis is all about: building chains (sugars are such chains or rings made of carbon) from individual chain links (e.g. from carbon dioxide).
So far so good. But how do you do that? And what do you need for it?
The answer is quite simple: you need an energy form and machinery that allow carbon dioxide to be built into a carbon chain.
Sunlight itself does not help at first: When photons, i.e. light particles, meet carbon dioxide, nothing happens. In any case, nothing that would be helpful in the synthesis of carbon chains. So when the sun is shining, something is not simply built up in the air.
In order to form chemical bonds, you need at least two things: first, favorable circumstances and second, something that is often described as a “power of reduction”. What do you mean with that?
Favorable circumstances here means nothing other than that a chemical reaction can actually take place on its own. It's basically a matter of probability
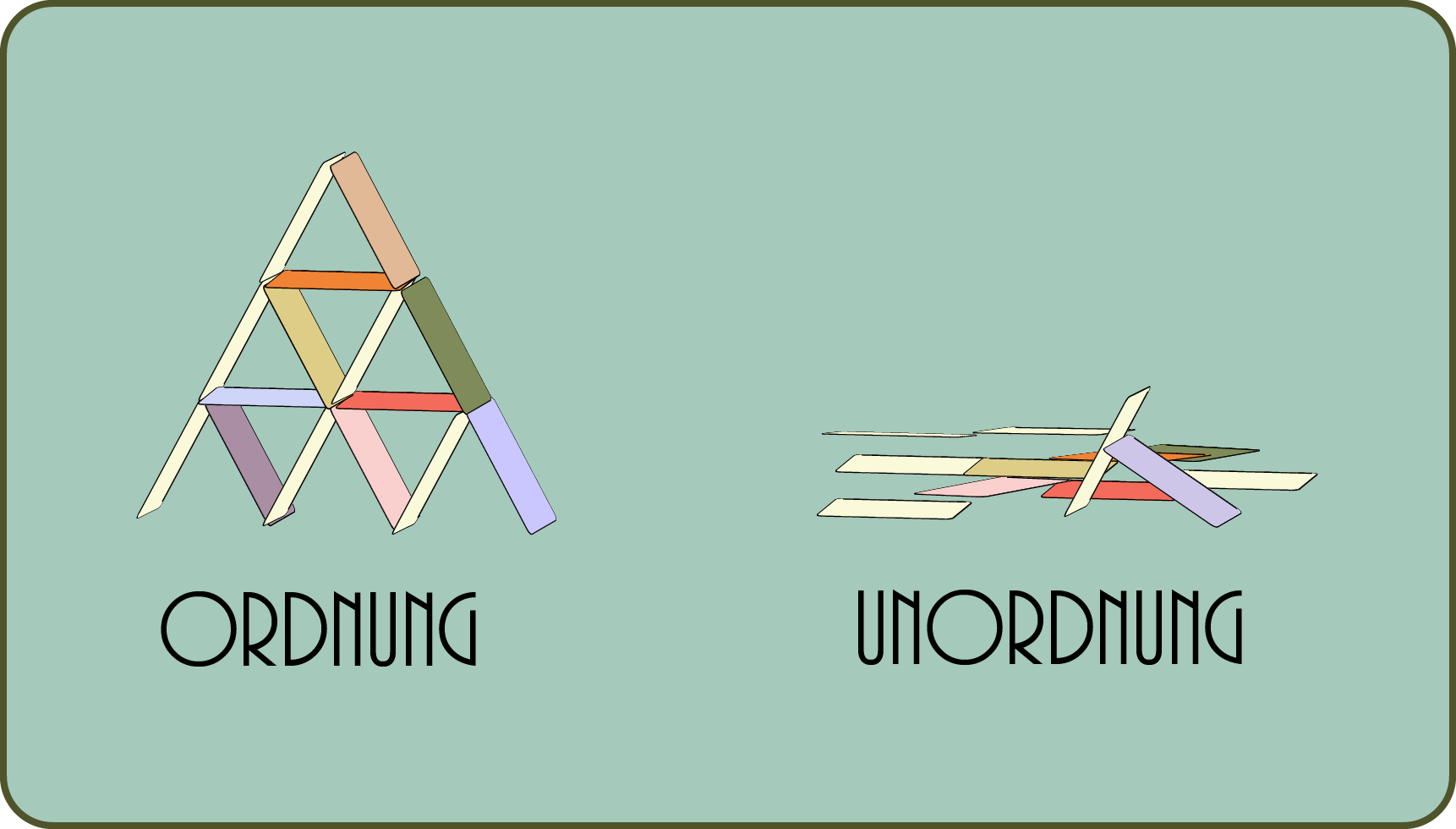
For example, a house of cards collapses almost by itself. The circumstances are favorable that this is exactly what will be happening.
Conversely, it is more than unlikely that a collapsed house of cards will rebuild itself by wind, is it? Therefore, chemical reactions have a direction, so to speak, that is, they follow a path in which the total disorder increases.
This applies not only to kitchens or children's rooms, but to the entire universe. In the end, the famous heat death of space is simply the most likely condition.
How can there be order at all? Well, by paying for the order in the small with greater disorder in the large.
In nature, every increase in order, for example the synthesis of a protein molecule from individual amino acid building blocks in a cell, is paid for with an increase in disorder, namely more heat in space.
But do not worry at first, because the latter is known to be huge and we will certainly have a few billion years left until everything is nice and warm.
Back to photosynthesis:
We said that to build carbon chains, you need “favorable circumstances” and not just sunlight:
So somehow you have to pay for this structure of order with disorder.
The currency, ie the "payment", is nothing more than a reaction that takes place voluntarily and with the release of heat (for example if you let a ball roll down a slope or burn petrol).
If you then couple it with a non-voluntary reaction (here, therefore, the building of carbon chains), you can force the actually non-voluntary reaction!
So this is what is meant by "favorable circumstances": the coupling of a voluntary reaction with an involuntary reaction.
And now watch out: The “currency” described above is a molecule in living cells that can drive just about anything: adenosine triphosphate, or ATP for short!
So ATP is a fuel: if it reacts with water, work can be done in the cell (for example, as a movement or as a structure of something).

Let us be clear: in order to increase order in the plant cell (e.g. to build up molecules for the next cell division), you need the fuel ATP. We will see later how photosynthesis can produce exactly this ATP.
In many cases, however, in addition to this form of energy (one would have to say more precisely, in addition to this generation of order in small and disorder in large), electrons are also needed to form new bonds. So now the reduction power described above comes into play.
What is “reducing power”?
The reducing power describes the willingness of a substance to donate electrons. Some substances give off electrons very easily, for example iron. Then it rusts. Other substances (e.g. gold) hardly ever donate electrons, rather they easily accept electrons compared to other substances. These and other “precious metals” therefore do not rust or only under very special circumstances.
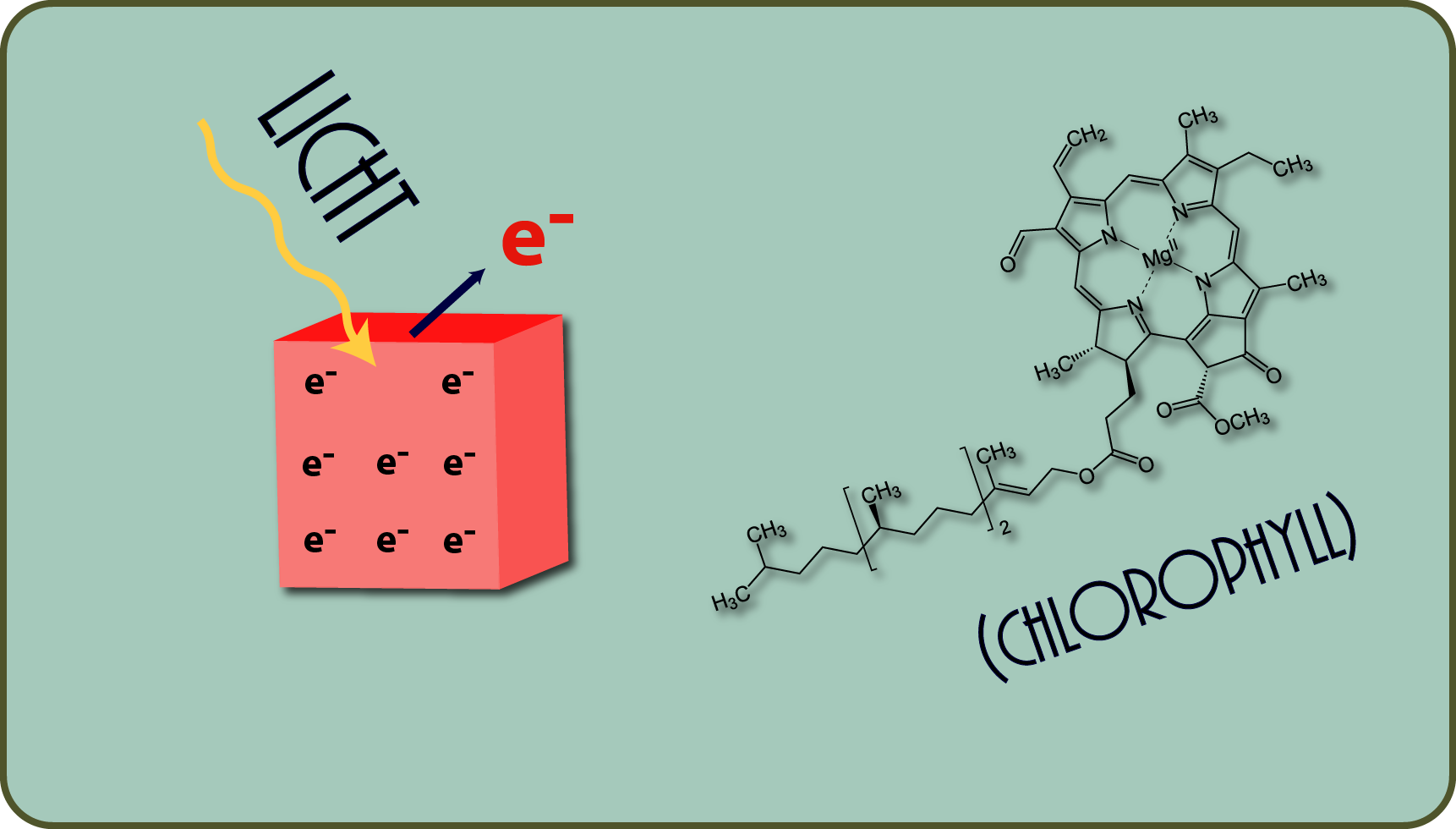
The electrons of a substance are also released more easily if they are excited by other energies (e.g. light). They then get into a state from which they can separate from their original atom and transfer to another atom.
And now it comes:
This is exactly what happens when light meets water during photosynthesis: the electrons of the water are transferred to other molecules in the green chloroplasts. This turns water: oxygen!
This does not just happen, but requires complicated machinery in the cell. Perhaps you now understand why it can be said that photosynthesis is the splitting of water by light!
Let's make it clear what the whole thing is about:
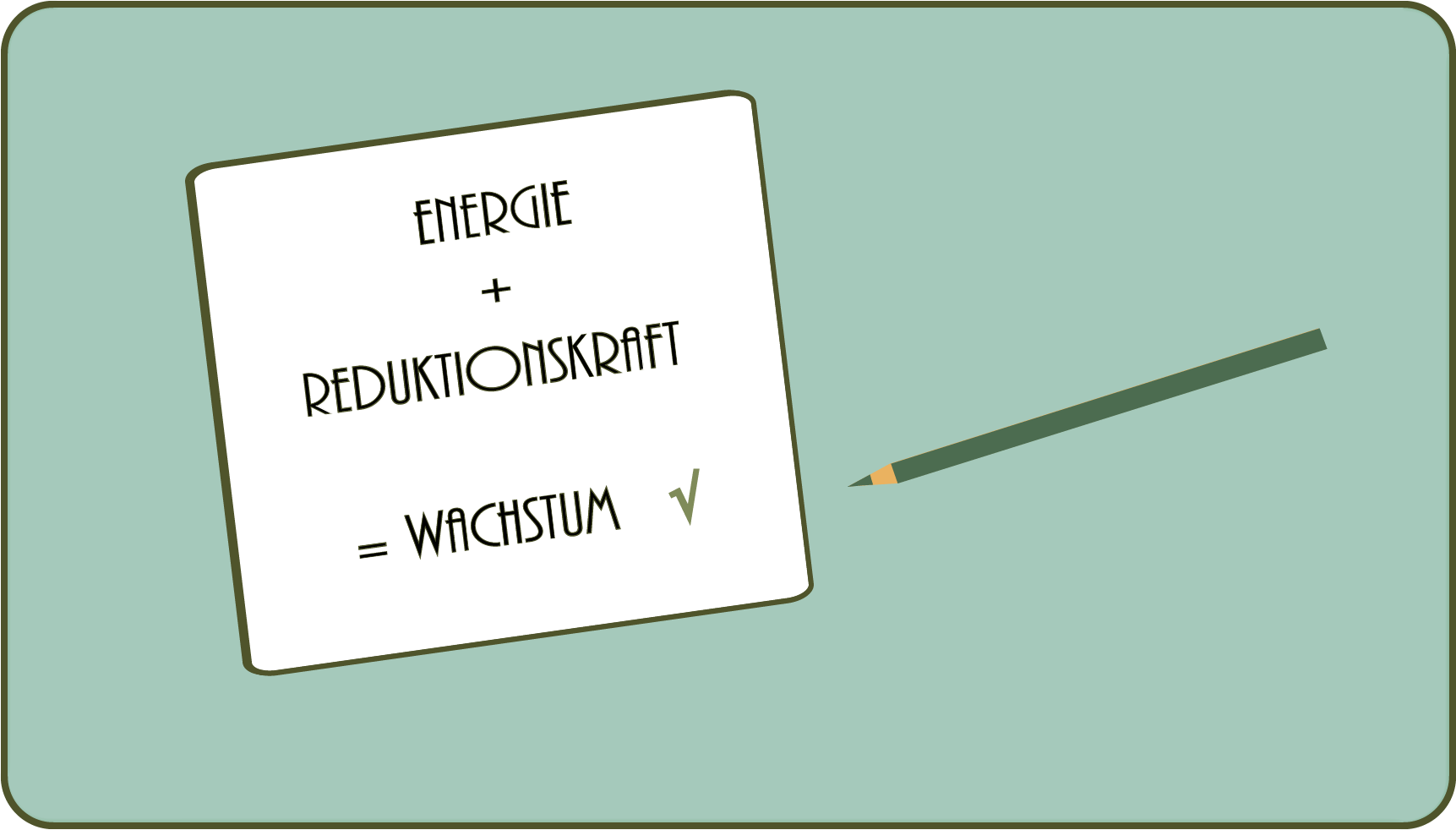
- To build connections, that is, to grow, you first need a fuel that helps make unlikely reactions possible: ATP is in the cell. All energy that is stored in the form of ATP ultimately comes from the sun (we disregard certain bacteria that can gain energy from minerals).
- You also need: Reduction power to transfer electrons, i.e. to form new bonds between atoms.
- Both, i.e. ATP and reducing power, are formed in plants by photosynthesis, in which light raises electrons to a higher energy level. This does not happen by itself, but requires, among other things. what makes plants green: chlorophyll!
- When water is split by light, electricity is generated: chloroplasts are machines, so to speak, that generate electricity from light.
Have you ever thought about what a solar cell does on the roof of a house? It generates electricity from sunlight! Solar cells don't look particularly pretty and don't exactly remind you of plants, but in fact a leaf does something very similar in sunlight: it generates electricity (namely the flow of electrons) that is used to do work or to charge batteries (see in the cell can be used differently, of course).
The reason why forests and meadows are so green is, as you probably already know, that a molecule (the chlorophyll) in the leaves can absorb certain parts of the white sunlight. But what is a molecule that only absorbs parts of the white light? A dye! √

The part of the light that is not absorbed by chlorophyll but thrown back is: green!
Why is that? Couldn't the entire color spectrum of light be absorbed by plants? Such leaves would actually be black for our eyes and there is in principle nothing against there being such a thing in nature. Apparently, however, the system, as plants use it today, developed at an early stage of evolution, in which the red and blue parts of the light are used in particular. The chlorophyll molecule may not be ideal in this respect, but it is completely sufficient. By the way, certain types of algae use molecules that can absorb light precisely in these “gaps”.
At the beginning we talked about the fact that fuel and reducing power are the two essential elements for the development of growth.
We also claimed that the reducing power is generated by light, which lifts electrons to a higher energy level. What is the current doing in this story? Well, it flows. And just as a river always flows down the valley, so do the excited electrons: Downwards, i.e. to a less strongly excited level. As they say, they are doing work, just like driving a water mill with a river, for example.
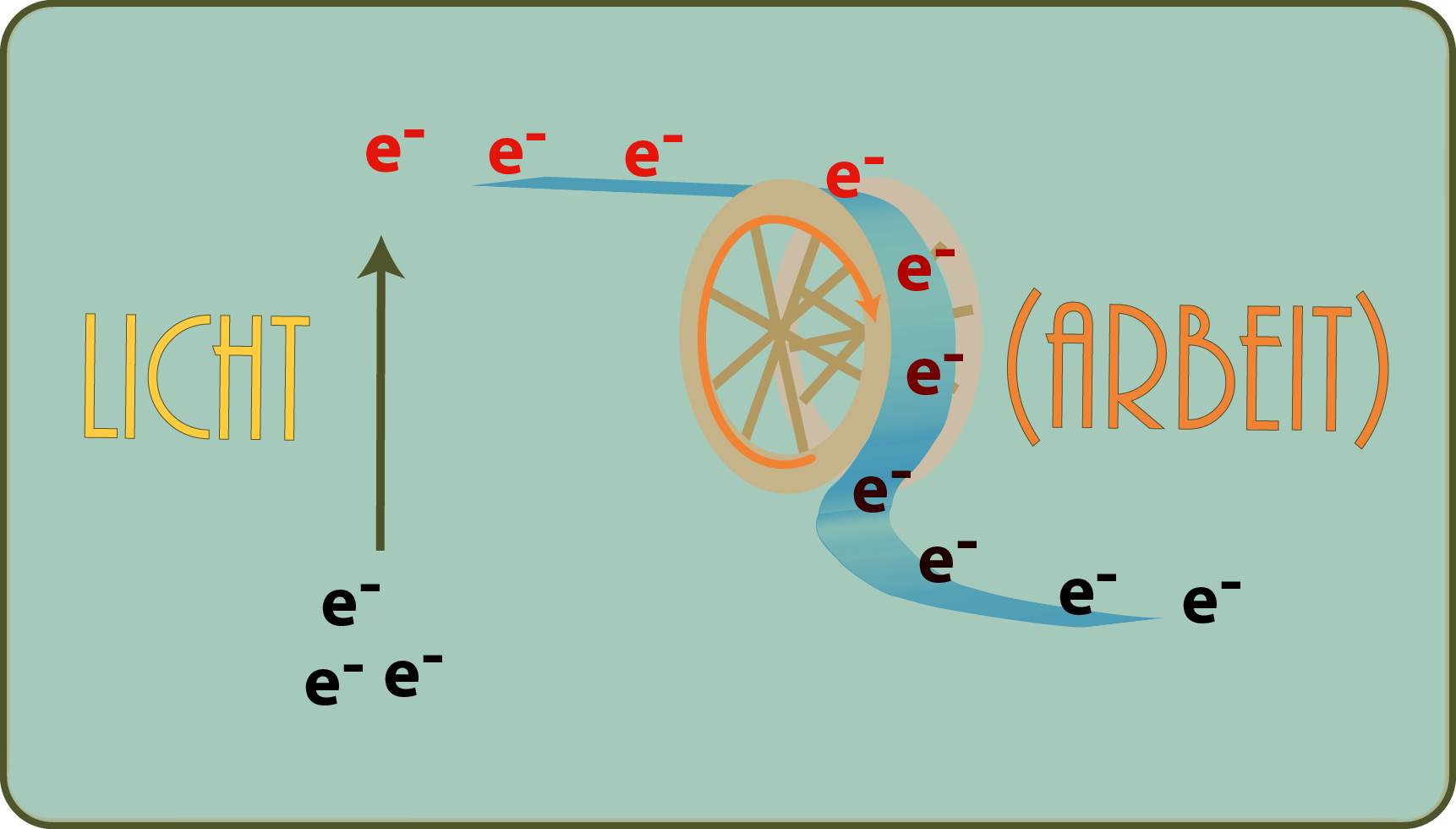
We will not go into detail here which complicated molecules are involved in these processes: Just as it is more important to understand the principle of a solar cell than to know its exact chemical composition, with the read you have everything you need to understand how sunlight can turn into wood, for example.
But, wait: is that all?
By no means, because what we have discussed so far is often described as a “light reaction” in order to distinguish it from another mechanism of photosynthesis, the so-called “dark reaction”.
2) Can you work with electricity in the dark?
The so-called "light reaction" is indeed light-dependent. For example, if you darken a plant leaf, oxygen is no longer generated from water. Nevertheless, the energy stored in the form of ATP and reducing power (i.e. excited electrons) can be used to build up molecular chains. So the whole thing works (at least theoretically) in the dark (therefore "dark reaction"), which does not mean that it MUST be dark! More on that in a moment.
What is the dark reaction about?
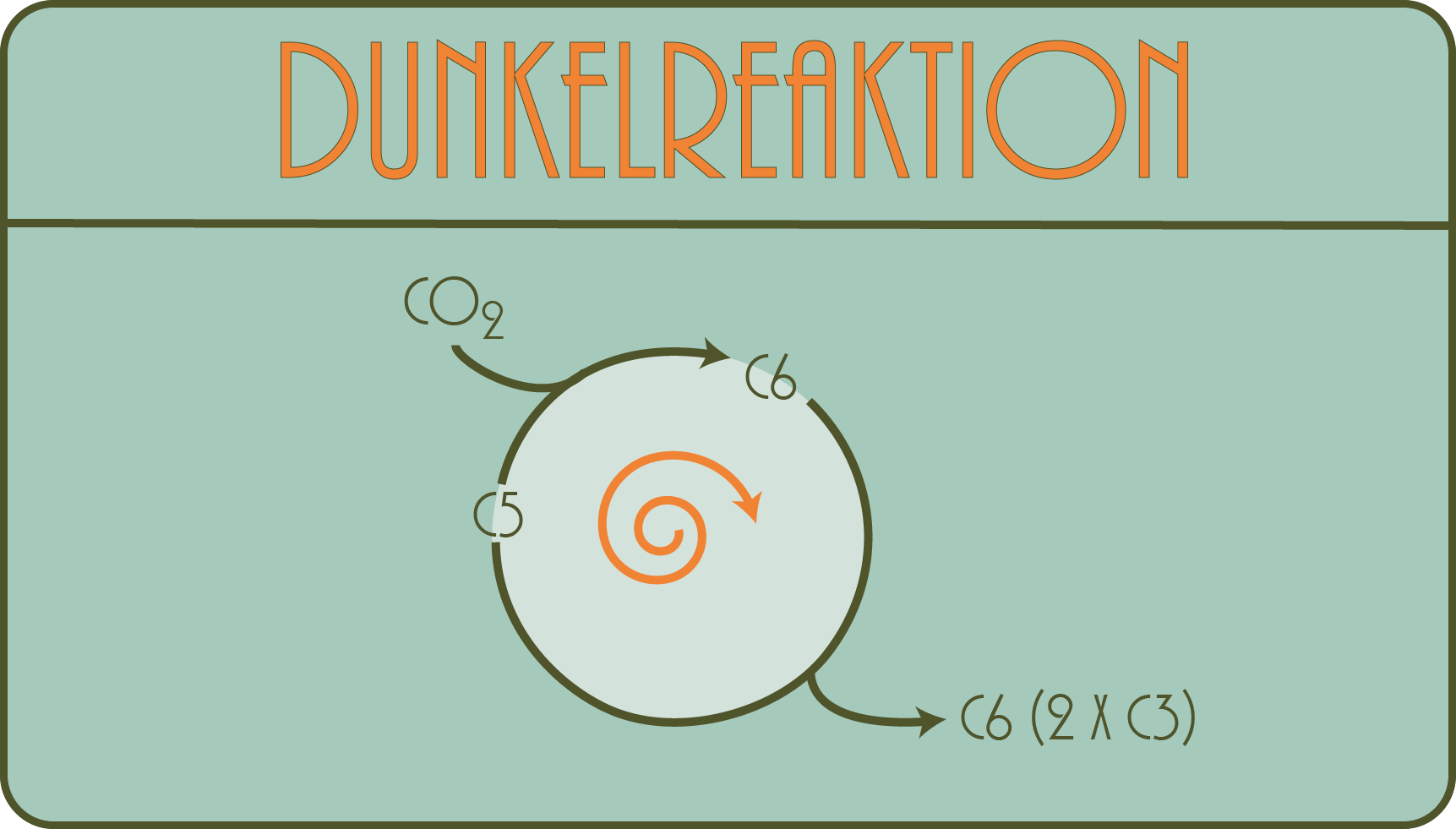
Something amazing happens here: a gas is converted into solids. So, so to speak, solid material is created from the air. A process that is still a huge challenge even for complicated processes and machines ( -> see eg Haber-Bosch method ). The gas we all know is, of course, carbon dioxide. We constantly exhale (and inhale) it.
In the dark reaction, a plant cell now manages to incorporate this gaseous carbon dioxide in a carbon chain, to "fix" it, so to speak, and to make it part of a solid (for example, sugar). Ultimately, all of the carbon in the sugars, proteins or fats of one (e.g. yours!) Body comes from the carbon dioxide in the air.
The process is extraordinarily complicated in detail (and is called the “Calvin-Benson cycle” after its discoverers, you will encounter it everywhere if you read something about photosynthesis), but the step that is essential for understanding is taken by only one , but mastered a very astonishing molecule, an enzyme that bears the long name "Ribulose-1,5-bisphosphate-carboxylase / -oxygenase" and is often abbreviated to "RuBisCO".
Astonishing for several reasons: firstly, it is the most common water-soluble protein on earth, and secondly, as said, it can incorporate a gas into a solid. Above all, however, from today's perspective, it can be said that it was enormously underestimated, especially by the researchers who initially examined it.
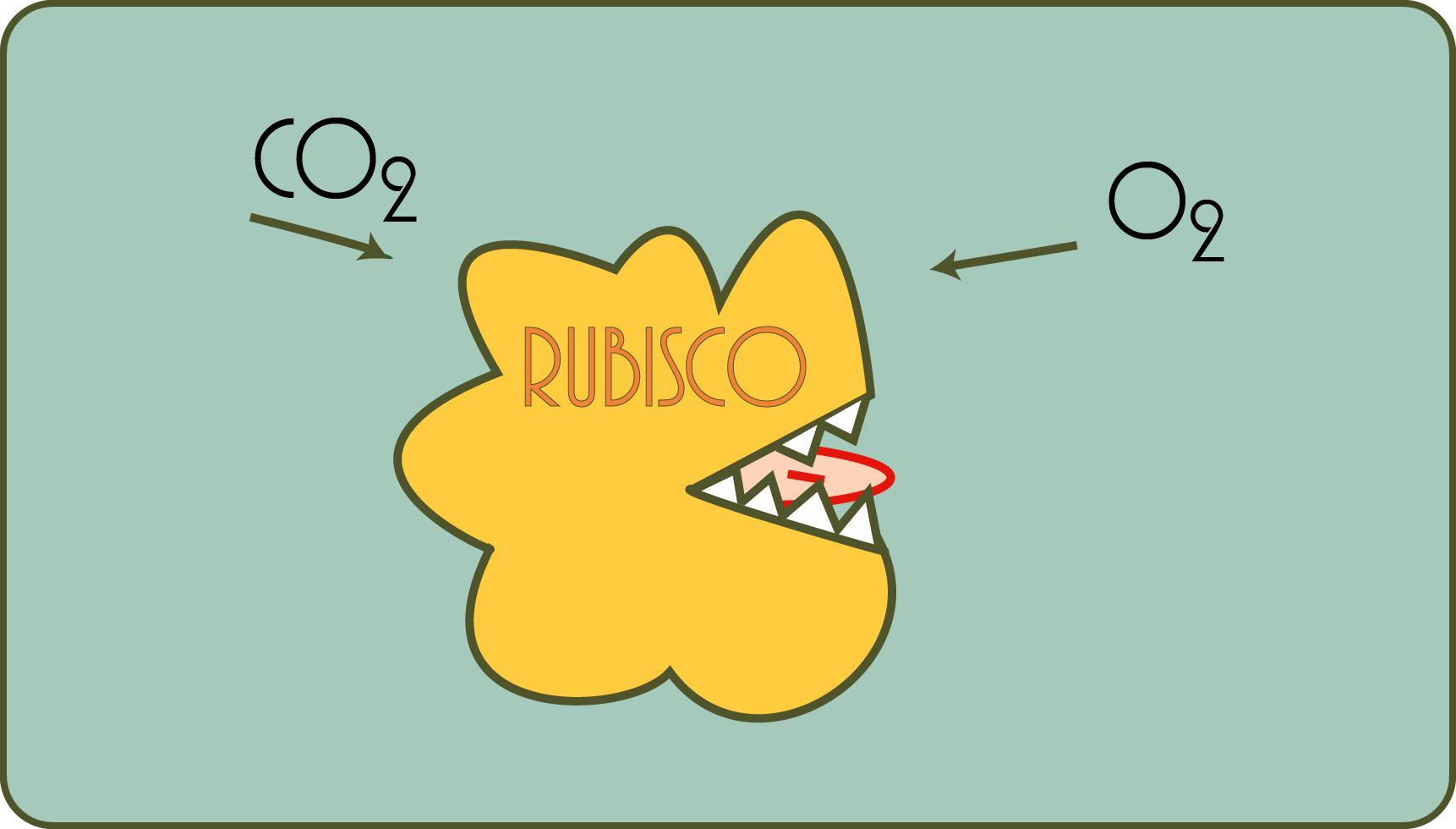
How did that happen? Well, soon after the discovery of RuBisCO, it was found that the enzyme was an astonishingly poor catalyst for fixing carbon dioxide: yes, enzymes accelerate chemical reactions and RuBisCO does not seem to be particularly good at this. At the same time, and to make matters worse, it also fixes oxygen to a significant extent instead of carbon dioxide! Strange, isn't it? This process is also called "photorespiration", so to speak, "light-dependent breathing". The plants have just produced oxygen, so are they consuming it directly? What sense is that supposed to make?
In fact, this process seems extremely wasteful. From today's perspective, this is best understood if one assumes that RuBisCO was created and was evolutionarily optimized when the oxygen concentration in the atmosphere was comparatively low.
Apparently the price of this waste seems tolerable for many plants, others have developed mechanisms to compensate for this disadvantage in an oxygen-rich atmosphere with a few biochemical tricks. Such plants are called "C4 plants" or "CAM plants", depending on which biochemical path the plants have taken.
One finds here, as incidentally in many places in nature, that the phenomena express their history and by no means function ideally (that is, as an engineer with sufficient knowledge might have constructed them). They are often a compromise that has to be made once a path has been taken and conditions change. To choose a corresponding picture: You can also arrive with a used and somewhat makeshift car, if perhaps a little slower.
Crazy world: The dark reaction can only take place in the light!
While entire generations of students have learned well that the described “dark reaction” can take place in the dark (or must it?!?), It is now known that the opposite is true: the Calvin cycle actually works in the dark or at night not, among other things because the activity of the enzymes required for this depends on the reduction power obtained in the light reaction!
In the test tube, individual steps of the cycle work very well without light: namely, if you simply add the reducing power in the form of a dissolved salt (called NADPH, but that's not so important at the moment).
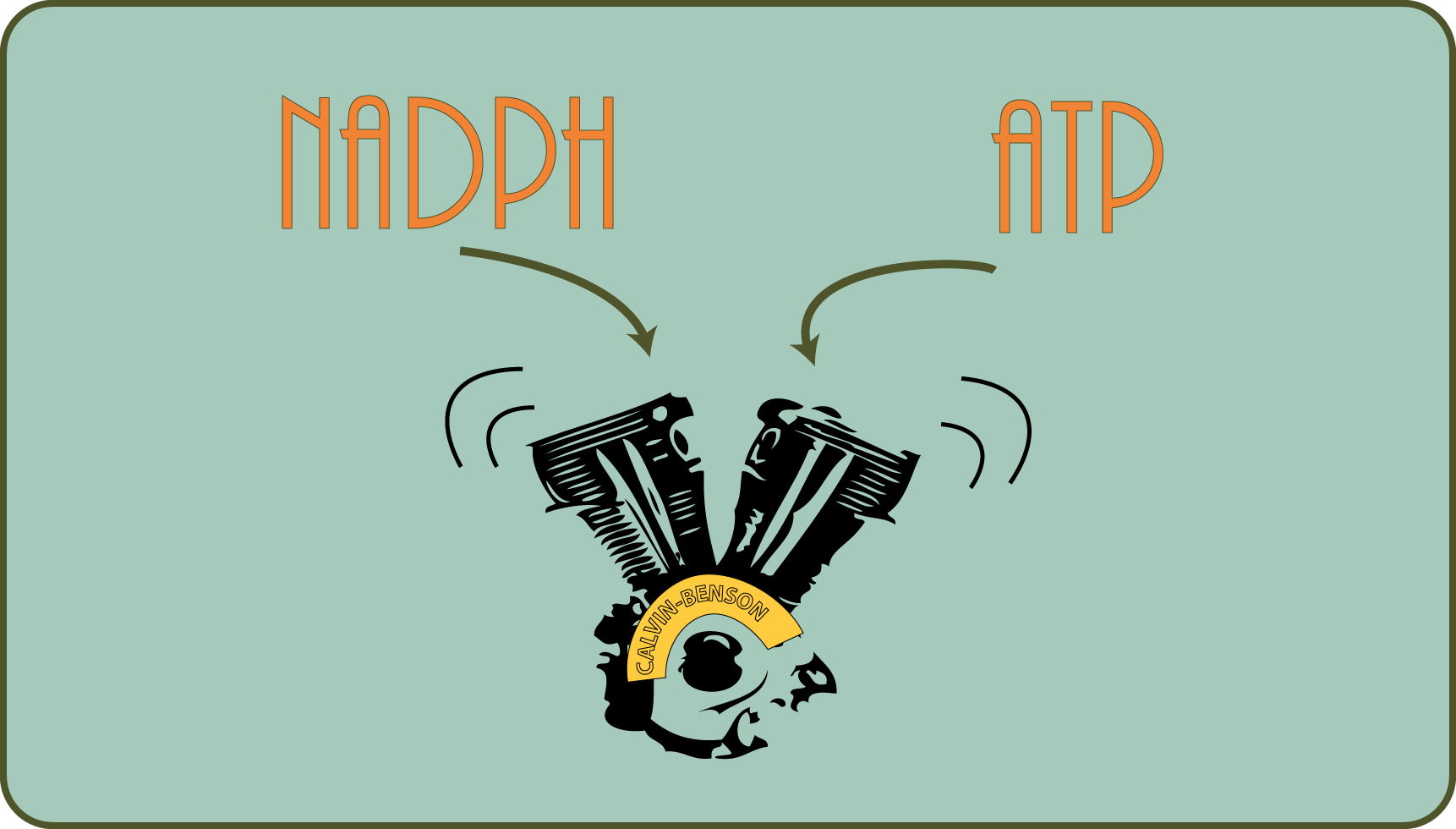
251/5000 What do we learn from this? Research is always on the move and what was true yesterday is no longer true today or tomorrow (freely based on Hannes Wader ).
Everything vibrates!
We now want to turn to an anatomical part of photosynthesis, so to speak, because we have already understood the following:
- Water is split by light using photosynthesis. It creates oxygen (which we breathe!), Hydrogen (whose electrons are available as a reducing force for the synthesis of carbon chains) and ATP (as a kind of universal energy currency).
- In a subsequent step, carbon dioxide is incorporated into existing carbon chains by an enzyme, RuBisCO. Here too, contrary to what the name “dark reaction” suggests, light is necessary.
- We have now understood what is necessary to turn sunlight into something that grows!
- We understood chlorophyll somewhat better as a dye that can capture light and somehow pass on this energy in the form of reducing power and ATP.
But what does that actually look like in the plant cell when light turns into a different form of energy (do you remember the solar cell?)?
Do not worry, now there are no complicated formulas. But you will realize what the principles are when converting light into another form of energy.
We can understand qualitatively what happens when a chlorophyll molecule is excited:
An example from music helps us here: Maybe you have heard of the phenomenon of resonance? This is about vibrations, vibrations that are released into the air, for example when a guitar string is struck and then oscillates back and forth. The vibration is transferred from the string to the air, which in turn causes your eardrum to vibrate in the ear, where the brain then perceives the whole thing as sound.
Vibrations can therefore be conveniently transferred from one system to another. For this, however, this system must also be able to vibrate itself! Such systems have a so-called "natural vibration", so once you hit them, they vibrate at a typical frequency (for example, a swing).
Resonance occurs when you stimulate this system with your natural frequency, i.e. when you push the swing exactly in the rhythm in which it would swing by itself after a single push. Then the swing system deflects much more than with any other excitation frequency. That's why you don't need a lot of strength to get a fat person up on a swing: just poke a little in the swing rhythm!
We come back to the guitar string. The same applies to them: if you, let's say with a tuba, generate a tone (i.e. an air vibration) that has the same frequency (i.e. the same tone, e.g. a low A) as the natural vibration of the guitar string, the guitar string becomes start vibrating with your own vibration without our intervention. It will probably buzz and create a deep A.
What does all this have to do with photosynthesis?
Well, it is the same here: Photons ("light particles") and electrons also vibrate! As you probably know, monochrome light, for example, has a very specific frequency. The electrons also have one, depending on where and in which molecule they are located.
The electrons of chlorophyll are now excited due to the physico-chemical properties (which we will not go into here: if you want to know more, read something about "conjugated double bonds") of this chlorophyll molecule by light of a certain wavelength (and thus also frequency) to swing. Namely, as we have already seen, of blue and red light.
Similar to how one could transport the fat child on the swing into the next tree top (no! Don't imitate!) By nudging on with the natural frequency of the swing, electrons can also reach a physically higher level through light. They actually move away from the center of the atom.
From "up there" you can now "jump" to places that were not previously accessible to you.
This is exactly what happens when we say that "reducing power" has been created. Spoken in the picture, it would be comparable to someone who was brought up to the tree by the swing and who can now jump on a seesaw on the ground with a lot of wums, where the next child (no! Don't imitate it!) Then rockets into the Air shoots.
Very practical: light energy funnel
How does one actually have to imagine that each light particle is actually immediately converted into a different form of energy?
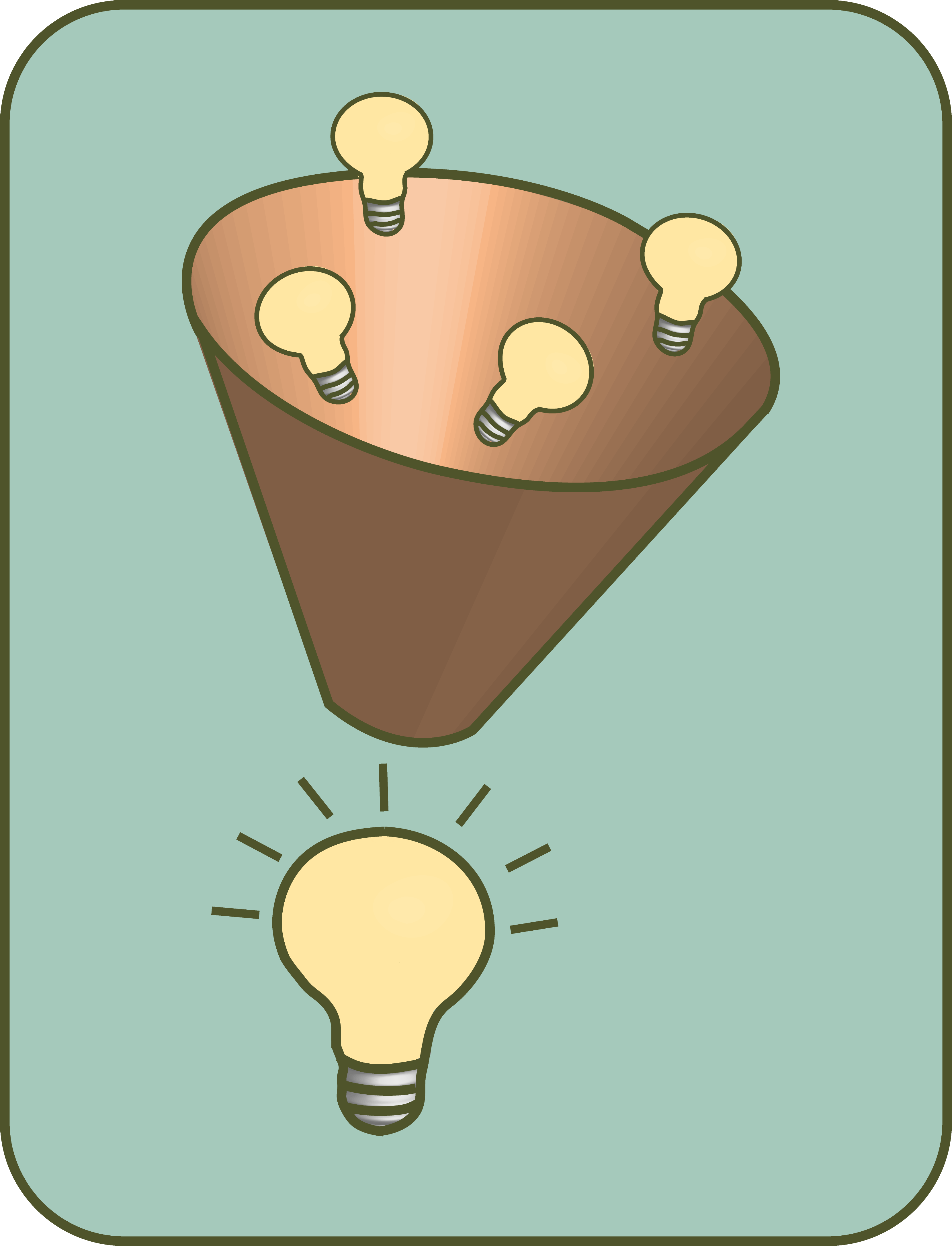
Here we encounter an interesting mechanism that serves to capture light and transport it from A to B within the plant cells. Plants have something that is reminiscent of a funnel, you could even say a trap. A light trap!
In this way, photons, i.e. light particles of different colors, are “caught” by different dyes. In addition to chlorophyll, e.g. So-called carotenoids (not only sounds like this, but actually gives the carrot its characteristic orange appearance).
These dyes act as antennas, so to speak, they absorb the photons and then pass on the excited state of their electrons to neighboring molecules. This happens until they end up in a so-called "reaction center" with a pair of chlorophyl molecules, which then conserves the original energy of the captured photon by irreversibly transferring its own electrons (and not just the excitation state) to another molecule.
The deeper meaning of this light trap is on the one hand to be able to capture light with a slightly larger spectrum than by chlorophyll alone, on the other hand a much larger area can be used to feed the photons to the limited number of reaction centers.
The best way to understand this is to imagine what happens when you stretch a cloth in the rain and put a small stone in its center. Where do you think most of the water ends up ?!
Finally:
So, that was a lot of stuff. Therefore we now repeat the most important things in a sensible order:
- Plant leaves collect light through a kind of light trap. The energy of the light particles serves to excite electrons. These electrons are then passed on to a place where they are available as a reducing force.
- The reducing power enables carbon dioxide to be incorporated into carbon chains using enzymes and ATP (the “fuel”). So: Light enables the increase of biomass = growth!
- The electron gap created by the transfer of the excited electrons in chlorophyll is filled up again by electrons from the water. This creates oxygen as a waste product from the photosynthetic splitting of water!
You have now understood the essential mechanisms of photosynthesis and can really have a say! You don't need to memorize chemical formulas at all.
Of course, the details of these mechanisms are very interesting! But they make sense especially if you have grasped the essence of photosynthesis. For example, if you just stubbornly memorize the Calvin-Benson cycle, you are quickly lost when it comes to recognizing the beauty and importance of these processes.
If you don't combine this knowledge with an Aha! Experience, you will have completely forgotten it after a short time, wanna bet?
We hope this article has helped you a little so that you can still say in a year: "I did understand what photosynthesis is all about!"
Kind regards,
your Kepler-Magazin team